Advantages and Disadvantages of Using Umbilical Cord Stem Cells
Advantages of Umbilical Stem Cells
Umbilical cord blood stem cells can be used to treat many different diseases and conditions. They are able to repair the body’s natural immune system, which is commonly damaged by chemotherapy or radiation therapy treatments. Umbilical cord blood (UCB) also contains hematopoietic progenitor cells that give rise to the various types of blood cells in the body including red blood cells, lymphocytes, neutrophils, macrophages and basophils.
Umbilical cord blood has also shown promise in helping treat many different diseases and conditions including leukemia, lymphoma, anemia and other genetic disorders.
Research shows that transplants from mismatched and discuss what you think about it! Advantages of UCB: The advantages of Umbilical cord blood stem cells (UCBSC) include their availability, safety profile and ease to collect by means of postpartum collection after vaginal delivery or cesarean section. Collecting UCBSCs does not require invasive procedures such as apheresis; therefore they do not cause any discomfort for mother and baby. Another advantage is that initially low cell dose can be multiplied many times in culture which leads to higher yields sought for transplant.
Disadvantages of Umbilical Stem Cells
Although they have shown great potential for treating several illnesses and injuries, there are still limitations with their use that need to be overcome before they will become more commonly used as treatment options. These include:
- one treatment is not enough to treat an adult with umbilical stem cells
- once transplanted, no more cells can be harvested from that source
- cord blood stem cells require more time to graft than bone marrow transplants
- no nationwide system for collection and storage of donated cord blood
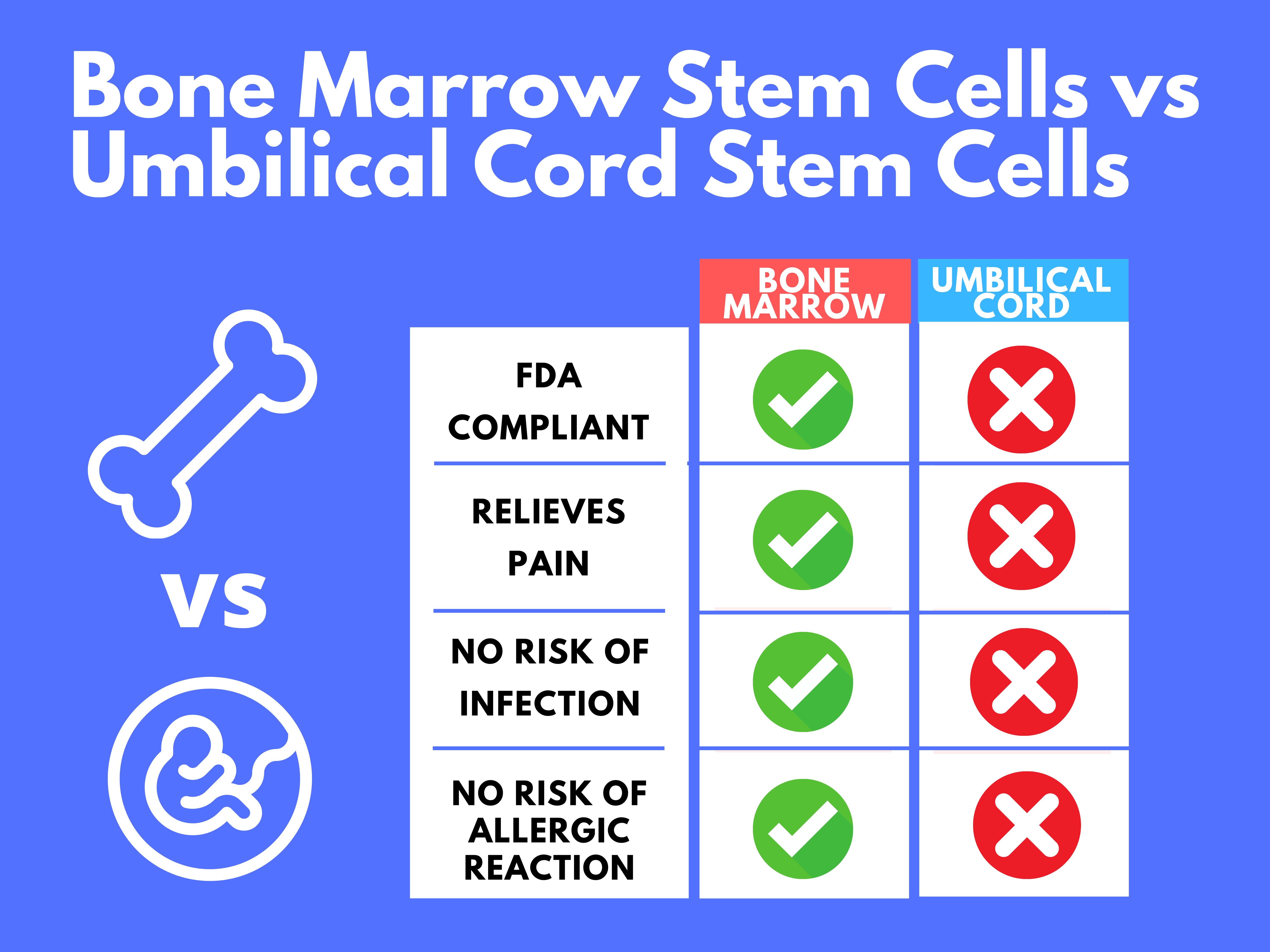
Bone Marrow Stem Cells vs Umbilical Stem Cells
Which are better to treat back and joint pain? It is a controversial topic. The answer depends on whom you ask. However, the devil is in the details [or facts]. I want to present facts so you can decide. I have summarized these facts in the table below and the detailed explanation follows.
Are Bone Marrow or Umbilical cord Stem Cells FDA Compliant?
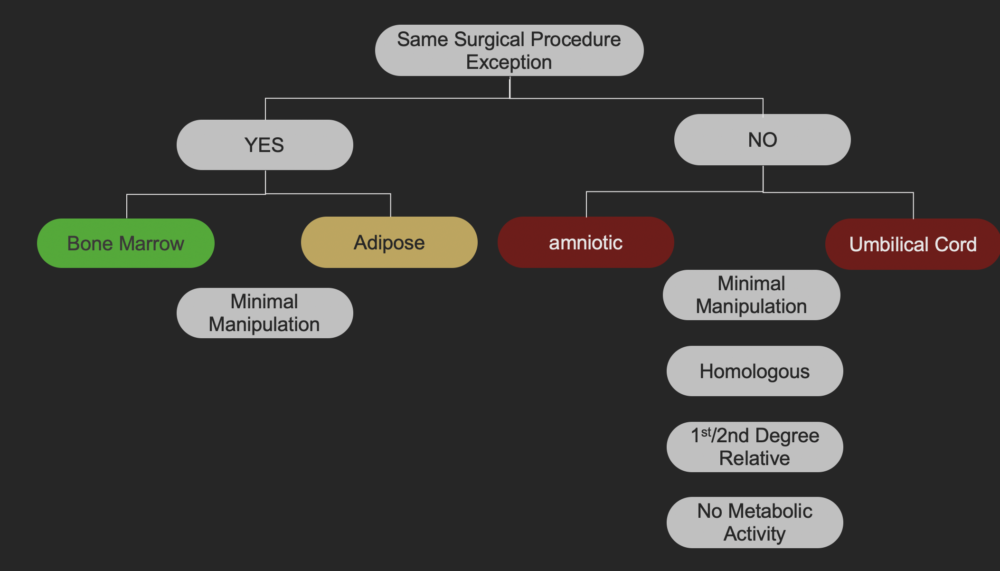
Based on the latest FDA guidance, Bone Marrow Stem Cells are the most compliant of all the commercially available stem cells. These cells fall under the “Same Surgical Procedure” exception.
What does that mean?
If a physician removes stem cells from the patient’s body and reinjects them on the same day, the procedure is called “Same Surgical Procedure”. Bone Marrow Stem Cells qualify for this exception and Umbilical Cord Stem Cells do not.
Why is this important?
If the stem cells qualify for the “Same Surgical Procedure” exception, the only criteria we must follow to be FDA compliant is the “Minimal Manipulation” criteria. This means that the stem cells should not be excessively tampered. When bone marrow stem cells are extracted from the iliac crest [back of the hip], we centrifuge them to concentrate [increase the number of stem cells per ml]. Centrifugation is considered “Minimal Manipulation” by the FDA. Hence, bone marrow stem cells seem to be FDA compliant.
Since Umbilical Cord Stem Cells do not meet the “Same Surgical Procedure” exception, they have to follow many other criteria in order to be FDA compliant. These criteria are…….
- “HOMOLOGOUS USE”. This means that the cord blood and cord tissue should do the same job as it does in the fetus. The cord blood is supplying nutrition to the fetus and cord tissue is supporting the blood vessels so they do not kink. So when this tissue is injected into the joints to treat pain, it is not performing the same function as it does in the fetus. This is one of the reasons why they are not FDA allowed.
- “CELLS SHOULD NOT HAVE METABOLIC ACTIVITY”. The number one reason to inject Umbilical Cord Stem Cells in the joints is to decrease inflammation and reduce pain. This cannot happen if the cells do not have metabolic activity. In other words, if the Umbilical Cord Stem Cells work, they are not FDA allowed. Shockingly, commercially available Umbilical Cord Stem Cells do not have any live functioning cells. I will explain this later.
- “ALLOGENEIC USE IN A FIRST-DEGREE OR SECOND-DEGREE BLOOD RELATIVE”. All of the commercially available Umbilical Cord Stem Cells are from another human being. Unless the patient is getting these cells from their first or second degree relative, these cells are not FDA approved.
The FDA guidance makes it very clear that the Umbilical Cord Stem Cells are not compliant.
Which has the most data regarding efficacy? Bone Marrow vs Umbilical Cord Stem Cells?
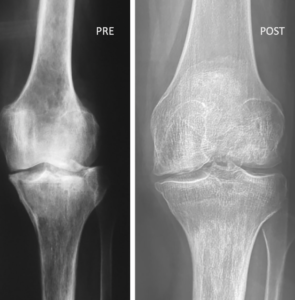
Dr. Phillipe Hernigou, an orthopedic surgeon in France has been doing stem cell therapy with Bone Marrow Stem Cells for more than 30 years. He has published numerous studies showing that Bone Marrow Stem Cells help many patients suffering from pain. In one of his landmark studies, he has shown that when compared to total knee replacement, Bone Marrow Stem Cells provided much better patient satisfaction rates. Moreover, there were more complications in patients who received knee replacement. He also showed regeneration based on x-ray films as shown below. These pictures are from his study.
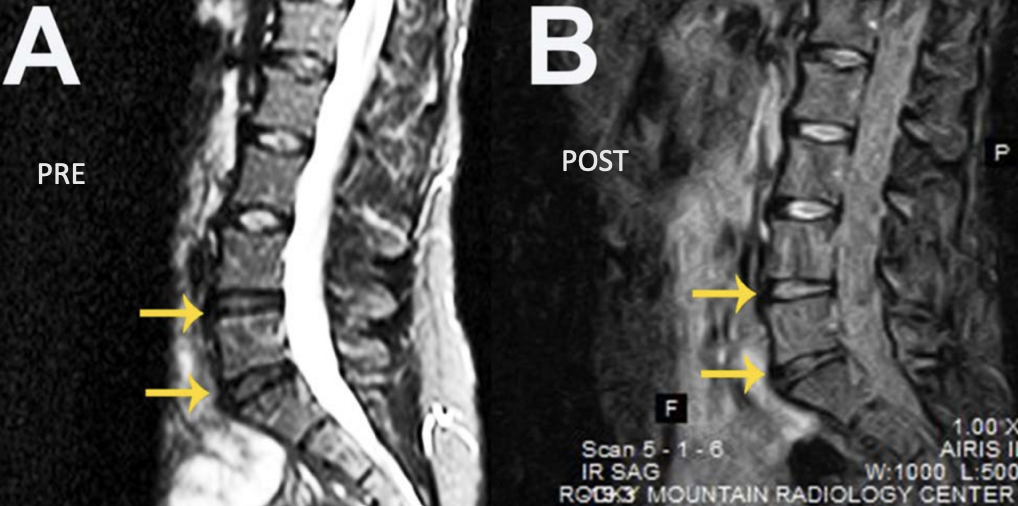
Dr.Pettine published another important study regarding Bone Marrow Stem Cells and back pain. He found that by injecting Bone Marrow Stem Cells into the lumbar discs, 81% of the patients obtained significant pain relief and 76% of these patients avoided fusion. He has also shown that these Cells can regenerate in the discs as shown below. These are the MRI pictures from his study. Yellow lines are depicting the discs and the disc in post [B] picture is more whitish indicating regeneration.
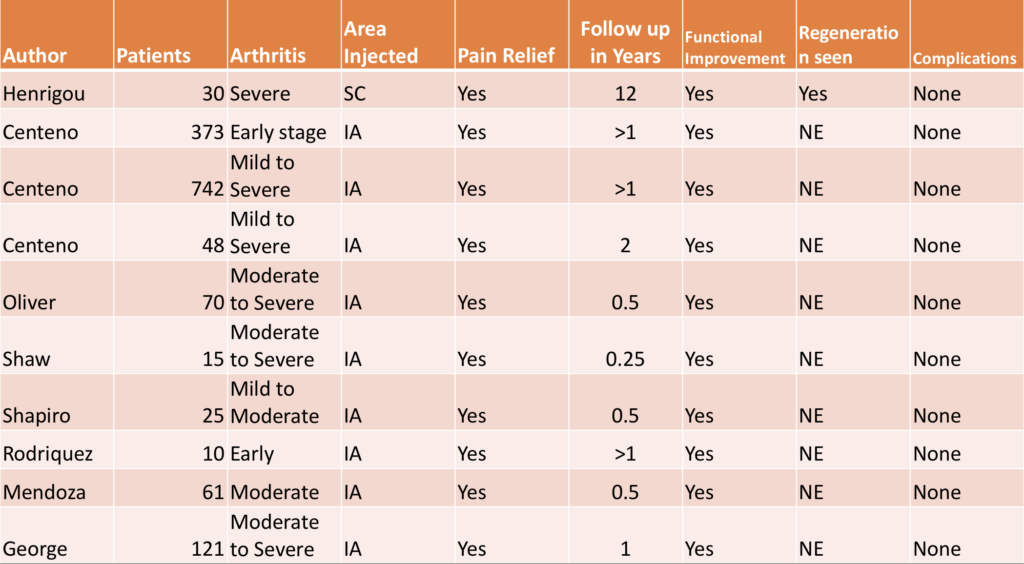
Here is a compilation of all the studies where bone marrow stem cells were used to treat knee arthritis pain.
Preliminary evidence shows that Stem cell therapy works. It also works long term. Dr.Hernigou’s study in the knees showed that even after 12 years, patients who got Bone Marrow Stem Cells were pain-free. Similarly, Dr.Pettine’s study revealed that even after 5 years, patients with back pain were pain-free. So how many clinical trials have been published about the efficacy of Umbilical Cord Stem Cells to treat pain?
The answer is ZERO.
Are Bone Marrow Stem Cells Safer vs Umbilical Cord Stem Cells?
As I mentioned earlier, Dr. Hernigou has been performing Bone Marrow Stem Cell procedures for more than 30 years. He recently published his experience and reported that there were no complications. Another large study by Dr.Centeno also echoed that Bone Marrow Stem Cells are very safe.
On the contrary, we know that the Umbilical Cord Stem Cells are not safe. Recently CNN and NY times reported 12 cases of serious infections when Umbilical Cord Stem Cells were used to treat pain. The infections were serious enough that these patients had to be hospitalized, some up to 2 months. The FDA has recalled the particular product which caused these infections. Since these infections were a result of contamination and poor donor screening, the CDC [Centers for Disease Control and Prevention] is asking these patients to be screened for Hepatitis C, Hepatitis B, and HIV.
Since Umbilical Cord Stem Cells are coming from other humans, there is a small risk of allergic reactions. HLA-A mismatch can cause GVHD [graft vs host disease] resulting from minor complications to serious events like death.
Commercially available Umbilical Cord Stem Cell products do not have Stem Cells. They have growth factors. It should be called Growth Factor Therapy and not Stem Cell Therapy.
Umbilical cord blood has very few mesenchymal stem cells. These cells are required to treat pain. They have a lot of hemopoietic stem cells which have no role in pain management. After birth, the umbilical cord tissue has to be harvested, cleaned, processed, sterilized, frozen, stored and transported to the clinic. It is difficult for cells to survive this chain of events. Cells, in order to be preserved, have to thaw in a systematic way over a period of 12 hrs. However, in the clinic, this tissue is “shock” thawed. This will kill the cells. Wharton’s Jelly [the soft tissue around the cord blood vessels] has a lot of mesenchymal stem cells. Sadly, because of the above-mentioned factors, the stem cells do not survive.
It is interesting to note that the product brochure of one the Umbilical Cord manufacturers clearly states that there are no stem cells in their product. This is a picture from their brochure.
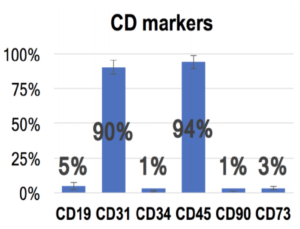
The above picture says that the Umbilical Cord product mostly has cells with cell markers CD 31 and CD 45. These are not stem cells.
Dr.Centeno’s group has compared the number of stem cells seen in the bone marrow aspiration samples and umbilical cord product samples. While they found a lot of stem cell in bone marrow, they did not find any stem cells in the umbilical cord.
Anecdotally, Patients do respond to umbilical cord therapies. That is mainly because they have growth factors. However, they do not have stem cells. As mentioned above, stem cell therapy gives long term relief. We do not have data to say the same about Umbilical Cord Stem Cells.
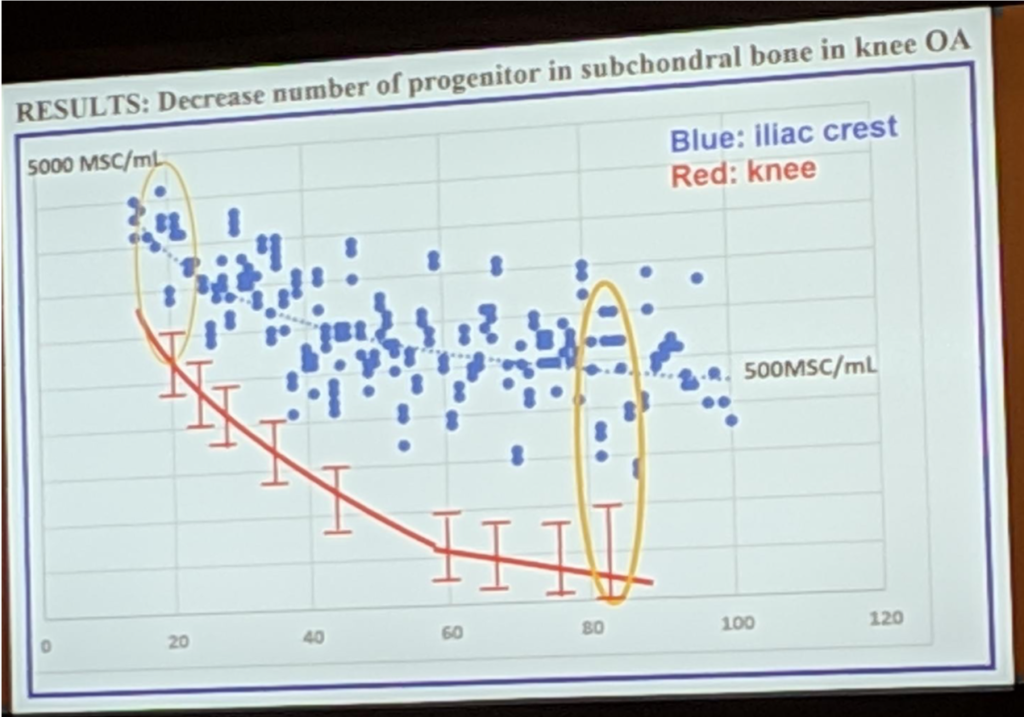
Proponents of Umbilical Cord Stem Cells say that as the patients age, the number of stem cells dramatically decrease in the bone marrow. This is true for stem cells in the marrow of long bones such as your femur [thigh bone]. However, we extract stem cells from the iliac crest [back of the hip]. Here the number of stem cells decrease but not as dramatically. There are enough stem cells even in the elderly. This is the reason why we continue to see good results in our older patients. In the picture, the blue dots represent the stem cells in the iliac crest as we age and the red line depicts the number of stem cells in the femur.
The other myth about Bone Marrow Stem Cells is that the extraction is painful. However, because of local anesthesia and sedation, most patients tolerate this procedure very well. See the video below.
In conclusion, patients should do their research before signing up for stem cell therapies. Interestingly, although bone marrow aspiration is time-consuming for the physician, umbilical cord stem cell pricing is usually higher. More importantly, it seems like an inferior product. It is your hard-earned money. Most importantly, it is your health. Spend wisely.